Brain and Immunity Lab
Research
My lab focuses on understanding the role of innate immune cells in the progression of Lewy body diseases. We utilize a combination of in vivo and in vitro models of synucleinopathies to uncover the interaction between innate immune cells and abnormal protein aggregates. Synucleinopathies include Parkinson’s disease (PD), Lewy body dementia (LBD) and multiple system atrophy (MSA), which are characterized by an accumulation of abnormally aggregated alpha-synuclein (α-syn) protein, which is the principal component of Lewy bodies. Extracellular α-syn aggregates act as a damage-associated molecular pattern (DAMP) for microglia in the CNS and also modulate immune cells in the periphery suggesting that central and peripheral immune responses play a role in synucleinopathies. My lab focuses on understanding how synuclein pathology affects central and peripheral immune responses and the role of innate immune cells in the progression of PD. The long-term goal is to explain how the immune system influences PD-associated brain changes, which may represent a novel mechanism and an avenue for treating neurodegenerative diseases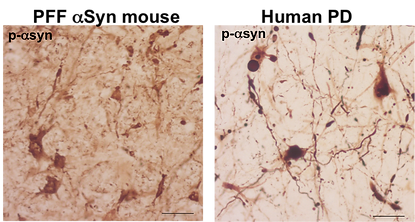 .
.
Lewy body-like pathology in preformed fibril (PFF) a-synuclein injected mice and Lewy bodies in human PD brains.
We utilize a combination of in vivo and in vitro models of synucleinopathies to uncover the interaction between innate immune cells and abnormal protein aggregates.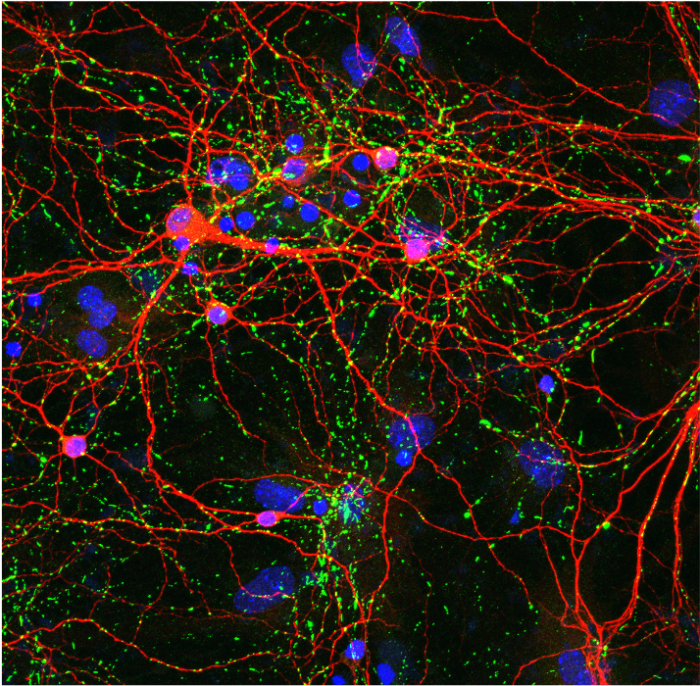 We have established the preformed fibril (PFF) alpha-synuclein (α-syn) mouse model exhibits many clinically relevant hallmarks of PD including dopaminergic cell loss, behavior deficits, and synucleinopathies. By utilizing this mouse model, we conducted a complete characterization of immune cell composition during a prodromal stage of the disease to determine whether CNS-initiated α-synucleinopathies alter immune cell profiles in the CNS and the periphery. Our study demonstrated that intracerebral-initiated synucleinopathies alter immune cell profiles not only in the CNS but also in peripheral lymphoid organs prior to neurodegeneration. Based on these findings, we investigate the crosstalk mechanism to link α-syn pathology in the CNS and its effect on the peripheral immune system. Primary mouse neurons with synuclein aggregates (phospho-a-synuclein (green), neurons (red), and nuclei (blue)).
We have established the preformed fibril (PFF) alpha-synuclein (α-syn) mouse model exhibits many clinically relevant hallmarks of PD including dopaminergic cell loss, behavior deficits, and synucleinopathies. By utilizing this mouse model, we conducted a complete characterization of immune cell composition during a prodromal stage of the disease to determine whether CNS-initiated α-synucleinopathies alter immune cell profiles in the CNS and the periphery. Our study demonstrated that intracerebral-initiated synucleinopathies alter immune cell profiles not only in the CNS but also in peripheral lymphoid organs prior to neurodegeneration. Based on these findings, we investigate the crosstalk mechanism to link α-syn pathology in the CNS and its effect on the peripheral immune system. Primary mouse neurons with synuclein aggregates (phospho-a-synuclein (green), neurons (red), and nuclei (blue)).
The long-term goal is to explain how the immune system influences PD-associated brain changes, which may represent a novel mechanism and an avenue for treating neurodegenerative diseases.
I. Elucidating the role of NK cells in synucleinopathies
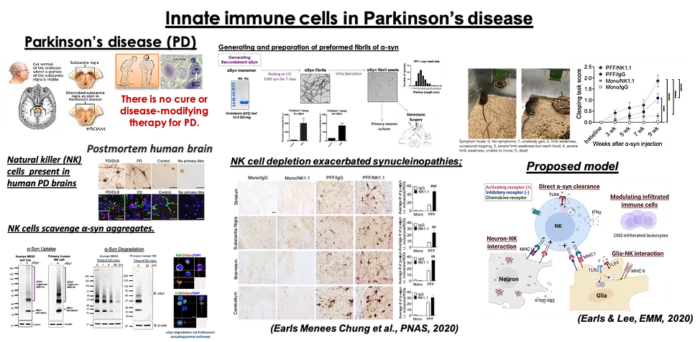
Natural Killer (NK) cells are innate immune lymphocytes serving the first line of defense. We found that NK cells infiltrate into the CNS prior to DA neuronal death. We also demonstrate that NK cells are able to endocytose α-syn aggregates in vivo depletion of NK cells in the PFF α-syn mice resulted in exacerbating motor deficits and increased synuclein pathology. The objective of our study is to investigate the mechanism (s) by which NK cells reduce α-synuclein burden, modulate inflammation, and exert neuroprotection. A comprehensive understanding of the novel role of NK cells in modulating synuclein pathology and motor symptoms could have a positive impact as a novel therapeutic for PD and other synucleinopathies.
II. Microglial RGS10 as a therapeutic target for Lewy Body disease
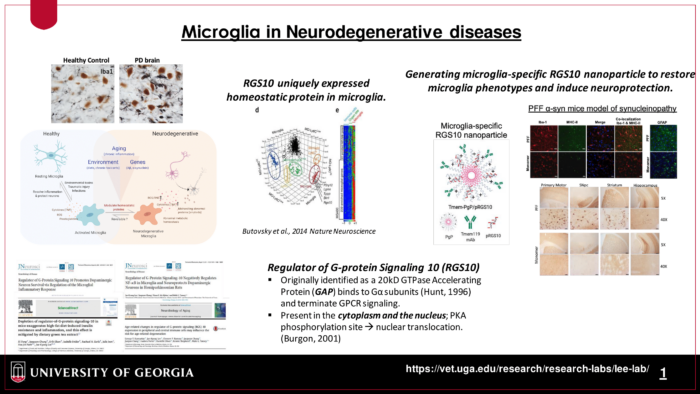
Age-related changes in inflammation and metabolism in peripheral tissues and the brain have been implicated as risk factors for neurodegenerative diseases. Importantly, inflammation associated with age and metabolic syndromes does not resemble the classical inflammatory responses that are the critical processes for normal tissue homeostasis, instead, it remains as low-grade, sustained in a long time period, and promotes damaging and can lead to various pathological conditions. However, the detailed mechanisms of how age-related inflammation and associated-metabolic changes affect the onset and/or progression of neurodegeneration have not been elucidated. Previously, I have identified the Regulator of G-protein Signaling 10 (RGS10) as a key regulator of microglia activation and neuroinflammation, and its neuroprotective effect on the nigrostriatal pathway. Our overarching hypothesis is that age-associated stresses reduce the level of microglial homeostatic protein, RGS10, consequently switches the phenotype to neurodegenerative microglia. We are currently investigating its role in age-associated chronic inflammation, metabolic syndromes, and cognitive deficits in the CNS. We also develop microglia-specific nanoparticles carrying RGS10 to restore microglial homeostasis to enhance amyloid fibril clearance and provide neuroprotection in synuclein-induced inflammation and neurotoxicity.
Lee Lab in the News
- ‘Natural killer’ cells could halt Parkinson’s progression
- UGA researchers report possible breakthrough in Parkinson’s treatment


